About Me
I’m a neuroscientist and postdoctoral fellow in the Departments of Organismic & Evolutionary Biology and Molecular & Cellular Biology at Harvard University. I study differences in motor behavior between two subspecies of deer mice (Peromyscus maniculatus). Deer mice that evolved in forests are more dexterous, and in particular, better at climbing, than deer mice that evolved in prairies. I’m trying to figure out how variation in their nervous systems causes this difference in dexterity. I hope that my work will help us understand more generally how animals, including humans, generate dexterous movements.
My work is supported by a K99/R00 award from the BRAIN Initiative. I previously received support from the Life Sciences Research Foundation and the Howard Hughes Medical Institute. I am a member of the Hoekstra lab.
I received my PhD in 2019 from the Biological and Biomedical Sciences program at Harvard University. In my dissertation project in the Gray lab, I investigated the relationship between neuronal activity patterns and activity-regulated gene expression. Before grad school, I was a research technician in the Gotoh Lab at the University of Tokyo where I studied cortex development.

Research Summary
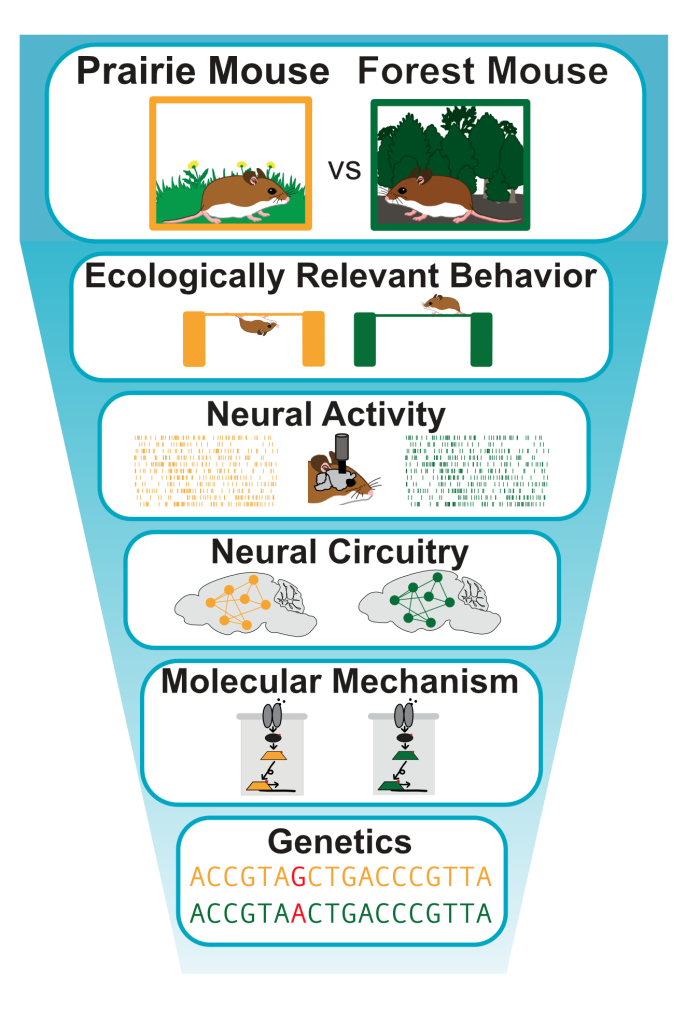
Ten thousand years ago, when glaciers receded from North America, they left in their wake a natural neuroscience experiment. While some deer mice moved into newly available forests and evolved dexterous motor abilities, including the ability to climb, others continued to live in prairies and never developed this dexterity. This adaptation occurred repeatedly in multiple forests across the continent, resulting in independent evolutionary replicates of the same dexterous behavior. I leverage the natural variation in behavior, neural circuitry, and molecular mechanism resulting from this evolutionary history to uncover the mechanistic bases of ecologically relevant dexterity.
While both molecular and systems-level bases of dexterous movement have been described, it has been difficult to develop a mechanistic understanding that spans functional levels. My work employs transcriptomic analysis, viral circuit tracing, high-throughput neural recording, and automated behavioral tracking in the comparative deer mouse system to identify and causally test the genetic, cellular, and neural mechanisms underlying ecologically relevant skilled motor behavior. Additionally, I compare the multiple evolutionary replicates of forest-adapted mice, to assess variation in the mechanisms supporting dexterity, identifying flexibility and constraint in nervous system evolution and function.
Corticospinal Expansion
A fundamental question in understanding nervous system evolution and function is: why does neuron number matter? Evolutionary expansion in neuron number, especially in the cerebral cortex, is thought to underlie cognitive ability. However, we don’t understand either the mechanisms by which this increase in neuron number is established or how having more neurons impacts the nervous system to alter behavior.
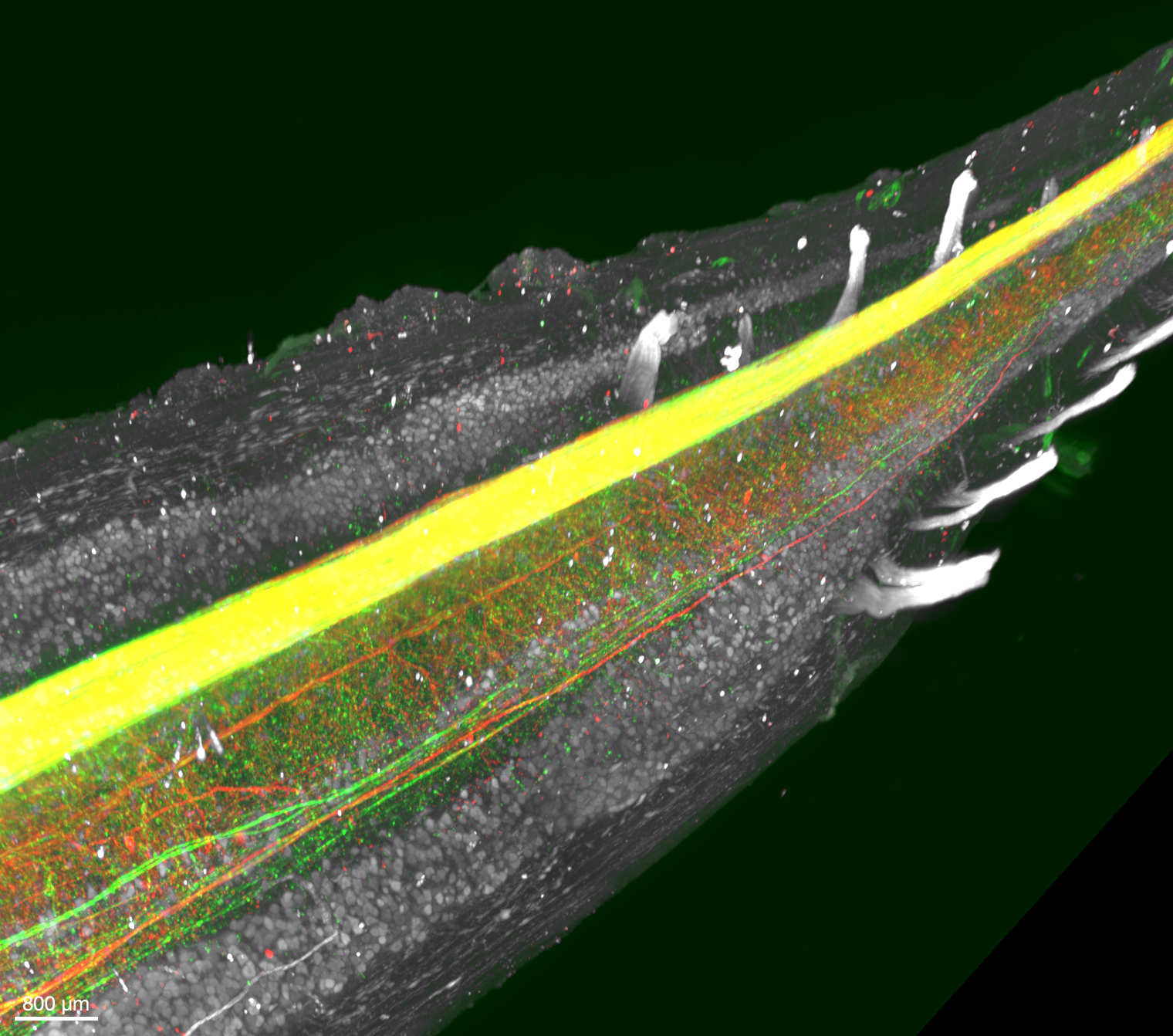
In our recent preprint, we show that forest deer mice have more corticospinal neurons and larger corticospinal tracts than prairie deer mice. These neurons, which project from sensorimotor cortex to the spinal cord are important for dexterous movement. Experiments across taxa have demonstrated that perturbing their function through lesion or optogenetics impairs hand dexterity and complex locomotion, like ladder walking. Furthermore, corticospinal system expansion is thought to underlie the exceptional dexterity of primates, including humans. However, it has been very difficult to test exactly how the number of neurons impacts circuit function and behavior. Excitingly, we have identified deer mice as a promising system to elucidate the role of corticospinal neuron number in behavior and circuit function. We find that corticospinal tract size is linked to dexterous climbing behavior in deer mice.
This work sets the stage for my research program aimed at understanding the causes and consequences of neuron number expansion. We have the tools to determine how genetic and developmental differences between forest and prairie mice result in differences in corticospinal neuron number, using an “evo/devo” approach. We will also determine how corticospinal neurons differentially impact circuit dynamics and behavior between subspecies by differentially integrating into the circuit . Given that the corticospinal system degenerates in Amyotrophic Lateral Sclerosis, and that several nerodevelopmental disorders involve irregular corticospinal neuron development, this work has the potential to generate discoveries relevant to human health.
Image above of a whole-mount cleared spinal cord, imaged by LIT International:
corticospinal axons are labeled via AAV injection into motor cortex
(green = secondary motor cortex, red = primary motor cortex), white = ChAT staining of motor neurons
Teaching and Mentoring
I have mentored undergraduates and full-time research technicians with a variety of skills and interests, from engineering to pre-med to computational neuroscience. I believe if you’re doing science, you’re a scientist! I build independence and project ownership with my students and mentees while balancing the need for support and guidance, taking into account differences in life experience and interest that students and mentees bring to the lab/classroom. My goal is to build confidence along with proficiency in experimental, communication, and quantitative skills.
Courses Taught
From Pipette to Pen [Nanocourse Director 2017-2018]
Statistics in RNA-seq Analysis [Instructor, 2017-2018]
Introductory Genetics for Graduate Students [Teaching Fellow 2014, Tutor 2018]
Advanced Molecular Genetics Laboratory [Teaching Assistant, 2011]
Ford Writing Tutor [2010-2011]

Outreach
I love talking science with everyone! Here are a few examples of my outreach work:
- Brains keep temporary molecular records before making a lasting memory. The Conversation. A summary of my 2018 paper.
- See me interviewed on Human Footprint on PBS!
- Pee is for pregnant: The history and science of urine-based pregnancy tests. SITN Blog.
- I also love visiting classrooms through Skype a Scientist! If you’re a teacher and want a scientist to talk to your class about neuroscience, behavior, evolution, or genetics—please reach out!
Image from me speaking at a Story Collider show at the Oberon Theater (RIP) in Cambridge, MA.
Publications
Authors in italics = mentees I supervised
Tyssowski KM, Cohen JD, Guo J-Z, Richardson PR, Cortina KE, Smith IH, Ejiogu DC, Hantman AW, Hoekstra HE. 2025. Evolutionary expansion of the corticospinal system is linked to dexterity in Peromyscus mice. bioRxiv doi: https://doi.org/10.1101/2025.10.16.682851
Tyssowski KM, Letai KC, Rendall SD, Nizhnik A, Gray JM. 2019. Firing rate homeostasis can occur in the absence of neuronal-activity-regulated transcription. Journal of Neuroscience, doi: https://doi.org/10.1523/JNEUROSCI.1108-19.2019.
Tyssowski KM, Gray JM. 2019. The neuronal stimulation-transcription coupling map. Current Opinion in Neurobiology, 59:87-94.
Tyssowski KM, Gray JM. 2019. Blue light induces neuronal-activity-regulated genes in the absence of optogenetic proteins. eNeuro, doi: 10.1523/ENEURO.0085-19.2019.
Tyssowski KM*, DeStefino NR*, Cho JH, Dunn CJ, Poston RG, Carty CE, Jones RD, Chang SM, Romeo P, Wurzelmann MK, Ward JM, Andermann ML, Saha RN+, Dudek SM+, Gray JM+, 2018. Different neuronal activity patterns induce different gene expression programs. Neuron, 98:530–546.e11.
*co-first, +co-senior
For a full list of publications, please click below.
Image credits
Top image by Andi Kautt.
About me portrait by Celia Muto.